GREEN ANTI-STOKES PHOSPHOR LED CIRCA. 1967 FROM THE U.S.S.R.
There are at least 31 images on this web page; somebody set up us the bomb.
Dial-up users please allow for slightly extended load time.
Last updated 10-20-2020
These amazing pictures show a very rare anti-Stokes phosphor green LED from Russia -- back then known as the USSR.
The LEDs are reported to have a Vf (forward voltage) of +1.70 volts at an If (forward current) of 10mA.
My electrical measurements:
Vf= 1.203 volts @ If=10mA
Vf= 1.325 volts @ If=30mA
My physical measurements:
5.30mm T
4.60mm dia. (not incl. flange)
5.40mm dia. (incl. flange)
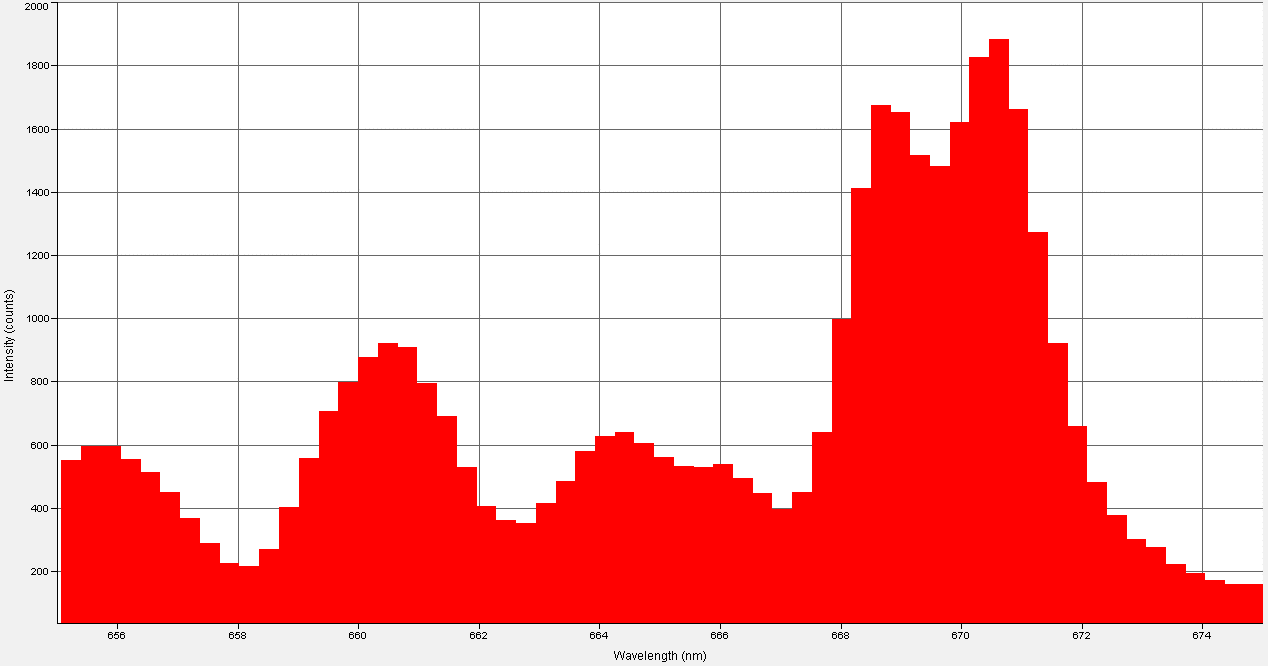
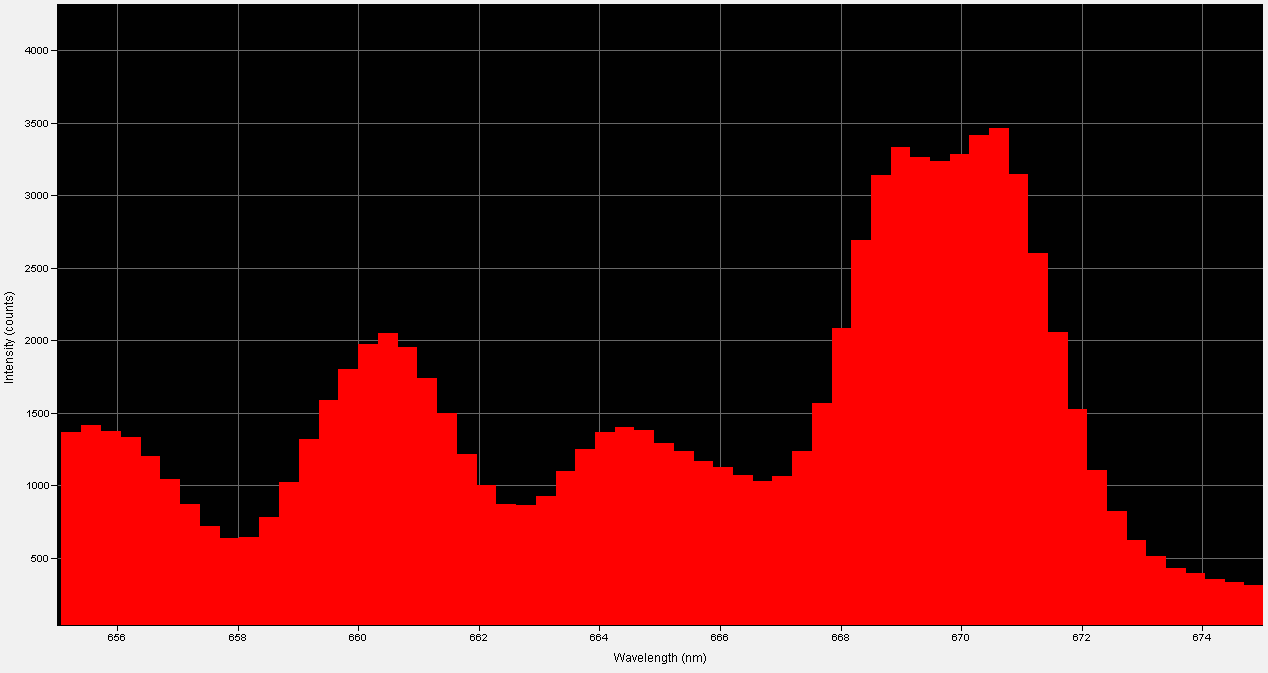
I measured the LED's, "out the front" power at less than 1mW at an If=150mA; this tells me that almost all of the LED's native NIR radiation is being absorbed by the anti-Stokes phosphor layer.
I have confirmed that my testing methodology is sound; I irradiated the same thermoelectric sensor used in the above test with a modern NIR LED at If=150mA and received a power output of 60mW.

Photograph of the LED itself.

Another photograph of the LED itself.
That "foggy" appearance is because I affixed some toliet paper to the camera's flash in attempt to mute it a bit; the flash's close proximity to the camera's lens resulted in some unwanted light "leakage".

Another photograph of the LED itself.
I took this one in direct sunlight and held a pair of +4 diopter reading glasses up to the camera's lens in effort to enhance focusing at near-macro distances.

Photograph "looking down the barrel" as it were with the LED being fed 2mA.

Photograph "looking down the barrel" as it were with the LED being fed 30mA.
This photograph was taken outdoors in shade plus I held a pair of +4 diopter reading glasses up to the camera's lens in effort to enhance focusing at near-macro distances.

Photo comparing this LED in size to a modern (2020) 5mm (T1¾) through-hole epoxy LED.
I must apologise for the furry pix; my last "good" camera was on a drone that was stolen approx. 14 months ago as of this writing (07-25-20); I was forced to use the camera inbuilt into my cellular telephone handset.