This is PAGE 1 of the 1960s exhibit. Go here for PAGE 2.
1960-1969: THE LED IS BORN
For a really great lesson about very early LEDs, go here, then keep reading here. :)
Another article about the invention of the LED that you may want to read is right here.
  
|
Our first exhibit: An example of technology used in the very first Light Emitting Diode ever mass-produced for industry and consumer. First appearing around 1969, this technology was actually available as early as 1962 but at that time only as a rather expensive and rare laboratory curiosity. Early models came in tiny metal cans with a clear window, bubble, or lens on the end. This model is obviously newer. This breakthrough model emitted infrared light, which is essentially invisible to the human eye. Fortunately for us, a variety of technology exists today which can capture this invisible light for old time's sake. Shown under the LED is one way of doing that: a chemically doped card that converts infrared into an orangish glow us humans can see. Video & digital cameras are also usually sensitive to this kind of invisible light. Chemically, this LED consists of the compound Gallium Arsenide; which is the metal gallium and the element arsenic, familiar to some of you as an older type of mouse & rat poison. Put two and two together, and you have the world's first completely solid-state, cold light source. Thus, the LED was born. |
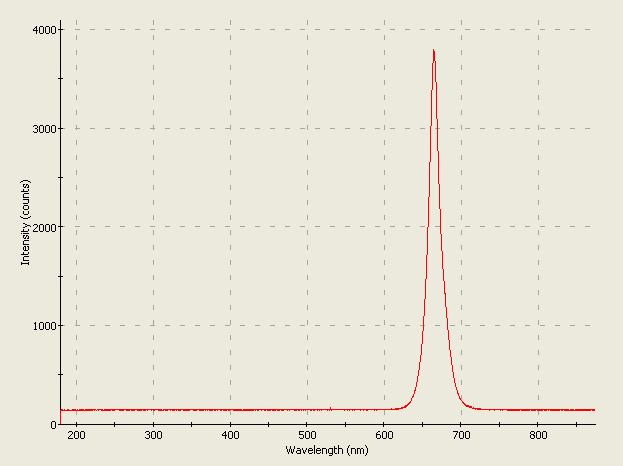
 This is how the LED would look to you if you could see infrared light. Here, the camera, sensitive to the 950nm wavelength, shows you the rather unusual double-whisker design of this early LED. An LED like this was probably first used in factory machinery for object detection; an upgraded version of this LED eases our daily lives, living in our TV and VCR remote control units and as sensors in all kinds of products we use every day. |
 
  |
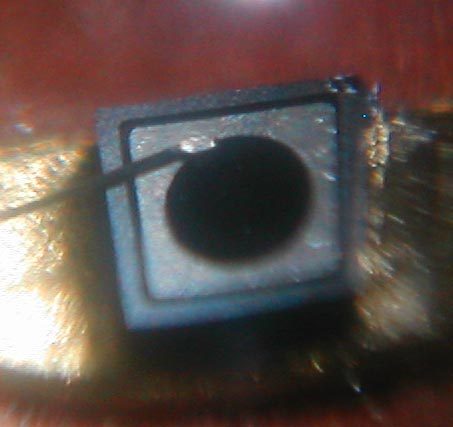
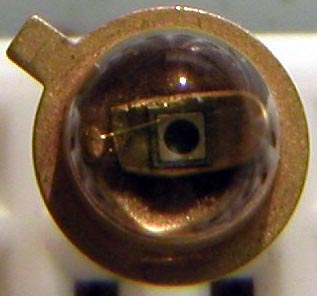
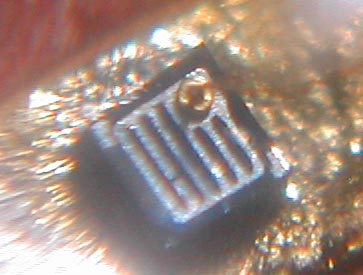
Arguably, this is likely one of the first visible red LEDs to surface. Appearing around mid 1969, this Monsanto MV2 red LED was purchased in London at a military surplus store on Tottenham Court Road. It originally cost ?1, at a time the typical wage earner made ?7 a week. So it was not cheap. As you can see by the picture, this came in a metal can with a glass or acrylic bubble on the business end. This LED has a very unusual chip construction, consisting of a gallium arsenide substrate (the base material) with a gallium arsenide phosphide emitting layer, and has a metal contact ring serving as the 1960s counterpart to today's gold bond ball. The actual light emitting surface is a circular region inside this ring, as shown in the picture. A small amount of red light also emenates from the vertical surfaces of the chip substrate; this is splotchy and irregular and is probably some GaAsP "overspray" of sorts from what was then a crude (by today's standards) manual manufacturing process. Chemically, this LED consists of the compound GaAsP on a GaAs substrate. When unlit, the color of the chip is a jet black. The anode ring is probably platinum, rhodium, or other nonreactive rare earth metal; and the rest of the metal components inside the LED are gold plated. Thanks to David Chambers in Scotland for hanging on to this LED for thirty plus years. |